Kits Turn CPAP/BiPAP Machines into Ventilators for COVID-19 Patients
In less than a month, engineers at Sandia National Laboratories converted 100 respiratory machines New Mexico hospitals had on hand into machines that can safely be used as ventilators to treat patients with severe cases of COVID-19.
BiPAP and CPAP machines are typically used to treat patients with sleep apnea and respiratory diseases. Continuous positive airway pressure (CPAP) machines apply continuous pressure to the airways. On the other hand, bi-level positive airway pressure (BiPAP) machines apply high pressure when the patients breathe in and low pressure when they breathe out. They also use a mask rather than intubating the patient like ventilators do.
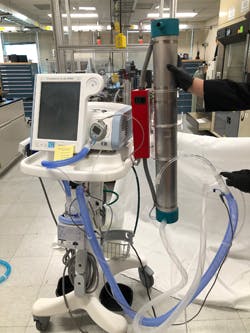
These machines cannot normally be used to treat patients with COVID-19 because they send the patient’s breath into the room, contaminating the area with virus droplets and putting medical staff at risk of exposure. So, a team of Sandia researchers developed pathogen management kits. The kits can be attached to respiratory machines and use UV to disable COVID-19 and other pathogens before a patient’s exhaled breath is circulated back into the hospital room.
Sandia partnered with Presbyterian Healthcare Services and the University of New Mexico Hospital to make sure the team was working to meet the needs of local hospitals. The hospitals provided information about the type and amount CPAP and BiPAP machines they had for conversion and what modifications would be most helpful for safely treating COVID-19 patients.
The project went from an idea through design, testing and validation within about three weeks. After a prototype of the kit was built and attached to a non-invasive ventilator, the team conducted biological and aerosol testing to ensure the design was safe and effective.
Sandia plans to transfer the pathogen management kit technology through a Cooperative Research and Development Agreement to a regional manufacturer to increase the production rate.
In addition, the team is designing an alarm to add to any ventilator to keep patients safe when non-standard equipment is used. It should also relieve some of the stress of healthcare workers handling too many patients by alerting them to any problems.
This project is one of about 50 that the Sandia staff is working on to help fight COVID-19.
About the Author
Stephen Mraz
Steve serves as Senior Editor of Machine Design. He has 23 years of service and has a B.S. Biomedical Engineering from Steve was a Flight officer in the U.S. Navy. He is currently responsible for areas such as aerospace and medical.

Leaders relevant to this article: