Hydraulic Fluids that Keep Going and Going
“Yeah, we’ve tried some ‘green’ fluids and we have had some bad experiences.” This is a statement I have heard more than once from clients who have jumped on the biofluid bandwagon before looking closely at what they were buying.
Over the past several years, global regulations have been passed requiring companies working in or near waterways to respect wildlife. In terms of hydraulic systems, manufacturing has traditionally used mineral oil and viewed it as a commodity. Yet, with growing requirements and restrictions, these manufacturers are being forced to use biofluids that are significantly more expensive without understanding the differences in fluid types, which can mean everything when it comes to performance.
Biofluid Classifications
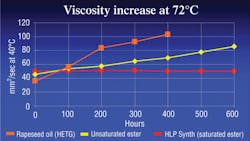
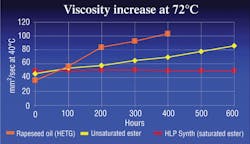
Biofluids can be broken down into four major classifications: HEPG, HETG, HEPR and HEES. HEPG fluids, or polyglycols, may be water-based but are not miscible with other hydraulic fluids. They are often incompatible with some seal materials. HETG fluids are plant or animal based and highly biodegradable. However, they generally cannot tolerate temperatures above 160°F for more than a few hundred hours. HEPR fluids, or polyalphaolefins, have good hydrolytic stability and biodegradability but may lose their viscosity as they run through a hydraulic system over time, see Figure 1.
HEES fluids, or synthetic esters, have good biodegradability. However, the wide variety of HEES products can perform drastically differently, depending on what type of HEES it is and its base composition. Unfortunately, HEES fluids are often generalized into one major category despite major differences in performance and longevity within the HEES class of products.
Saturated vs. Unsaturated Synthetic Ester
Esters are formed by condensing, or combining, an acid with an alcohol, a process called esterification. Just as not all fluids are created equal, neither are all synthetic fluids. All synthetic ester products can be broken into two categories—saturated and unsaturated. The saturation of a fluid is based on the chemical bonding within the fluid itself.
Oxidation, or aging of a fluid, is caused when a fluid reacts with oxygen. The result is extreme thickening and gumming of the fluid, along with deposits and shellac, which lead to major catastrophic system failures. Chemically speaking, an unsaturated ester has many open bonds that react with oxygen and cause the fluid to age more rapidly. Saturated esters, on the other hand, have significantly fewer open bonds, so they do not oxidize rapidly and will last much longer when subjected to high temperatures, Figure 1.
So how can you tell the difference? The easiest way to tell the difference is to ask the fluid manufacturer for the iodine number. This number identifies the number of open bonds available in a fluid. The higher the Iodine number, the greater the number of bonds that can interact and oxidize. Generally speaking, saturated synthetic ester products have an iodine number of less than 15.
Hygroscopic Properties of Fluids
Water content in an oil is largely influenced by temperature, relative humidity and chemical composition of the oil itself. Typically, the concentration of water varies from 300 to 800 ppm, where 0.1% is equal to about 1000 ppm. Any oil can take in and dissolve water to an extent. When fluid is mixed with a small amount of water — about 100 ppm (0.01%) — the fluid’s appearance will not change. It will appear to be transparent because water is dissolved at a molecular level, and even over time the oil and water will not separate.
However, all fluids also have a saturation limit, the maximum possible concentration of dissolved water in an oil. Once the molecules in the oil can take in no more water, the fluid is considered saturated. Any water beyond the saturation limit does not bind to the molecules in the oil and it becomes free water. You will often see this water sink to the bottom of a container while the oil floats to the top. It is extremely important to remove free water from any hydraulic system because it will hinder lubrication of internal parts (pump pistons, etc) and reduce life span of the oil.
Hydrolytic Stability
Hydrolytic stability, a fluid’s resistance to decomposition in the presence of water, is important to consider with biofluids. For equipment operating near waterways or hydraulic systems prone to condensation in the reservoir, hydrolytic stability is extremely important. Users need a fluid that is stable and will not break down or alter its composition when a small amount of water is present in the system.
A good example of a substance with good hydrolytic stability is table salt. Salt can be dissolved in water and recaptured later by evaporating the water. In this example, salt does not change its chemical properties when in contact with water. Edible oils do not behave the same way. When these oils are cooked with water for 500 hr at about 200°F, they become cleaved (bonded), forming new chemicals. This process is called hydrolytic fat cleavage, and it alters the oil’s chemical composition. Unlike the salt example, this change cannot be undone without a complex chemical process.
How Much Water is Too Much?
A common misconception being propagated in the field is that some fluids can handle high amounts of water before reacting. This implies it is acceptable to leave high amounts of water in a hydraulic system. Simply because a fluid claims a high level of stability when water is introduced does not indicate that the hydraulic system will function well with that amount of water ingression. The amount of water content required before a high-quality saturated synthetic fluid will begin to react is so significant that it is very likely that the system will begin to suffer problems from the water itself before hydrolysis occurs.
An analogy is the human blood alcohol level. In the state of Texas, the legal limit for blood alcohol content (BAC) is 0.08, meaning 0.08% of a human’s blood, by volume, is alcohol. A 200-lb man with a BAC of 0.04 has consumed two to three drinks, feels relaxed, and has some impairment of concentration but can still legally drive. This parallels the concept of water in a hydraulic system, where a small ingression of water will have minimal impact on the system and is still acceptable, but not ideal.
The ideal scenario is certainly no alcohol in your blood when you are behind the wheel, or in our case, no water in the oil. At 0.08, the driver in our example is now over the legal alcohol limit to drive. Likewise, our hydraulic fluid has now lost some of its lubricity, resulting in scarring, pitting, and corrosion of component internal surfaces.
At 0.20, the man now has a significant amount of alcohol in his system and experiences severe loss of motor skills and consciousness. Merely because the blood itself can handle significant saturation of alcohol does not necessarily indicate that the body can tolerate it, and the body will eventually shut down.
Our hydraulic system is not unlike the man in our example. The components in a hydraulic system are designed to run optimally using oil, not water. Excessive water in a hydraulic system can and will lead to major cavitation, corrosion, and catastrophic failure of the system.
According to the manufacturers of hydraulic components, what are the recommended standards for water content? Sauer Danfoss’ published literature states, “…the water content for continuous operation must not exceed 0.10% (1000 mg/kg). The lower the better. In principle water is a harmful contaminant, reducing the life of the hydraulic fluid and the mechanical components.” Bosch-Rexroth agrees, stating “Water in the hydraulic fluid may result in wear or direct failure of hydraulic components…During operation, the water content in all hydraulic fluids…must constantly be kept below 0.10% (1000 ppm).” Eaton’s Hydraulics Group also encourages its users to keep their systems free of significant water ingression. “It is recommended that for optimal life of components, water content in a hydraulic system should not exceed 0.07% (700 ppm). Beyond this water content, it can be expected the life of components and fluids will be reduced,” says Thelma Marougy, of Eaton Hydraulics.
The amount of saturation a hydraulic fluid can handle varies dependent upon the base fluid used and the temperature. The percentages above were established because problems related to excessive water — such as microbial growth, pressure rise, and swelling of filters — are often seen as starting at these concentrations.
Putting Fluids to the Test
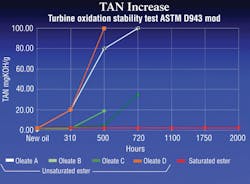
The most common tests for the life of a fluid are the RPVOT (ASTM D2272 — Rotating Pressure Vessel Oxidation Test), the Cincinnati Milacron Test (ASTM D2070) or TOST (ASTM D943). In these tests, fluids are subjected to the worst conditions possible to force them to oxidize. The tests monitor fluids for losses in oxidation resistance and increases in acid levels, as seen in Figure 2.
The TAN, or total acid number, is a common test that indicates the amount of deterioration of a hydraulic fluid. A high or increasing TAN indicates that the fluid is oxidizing — hydrolysis is taking place. Monitoring the TAN in a hydraulic system can provide an early indication of a potential problem and allow preventive maintenance to be conducted on the system as needed, saving both time and money.
In terms of standard mineral oil, an increase in TAN is always a bad thing. However, this is not always true with synthetic ester fluids. Synthetic lubricants can have acidic components that lead to corrosion, but some acids can act as corrosion inhibitors. The TAN for mineral oil based fluids should not exceed 2 mgKOH/g, but the TAN for saturated synthetic alternatives may reach 5 mgKOH/g without leading to any problems. These values will vary and should be verified with the fluid’s manufacturer.
Providing a Solution
Panolin HLP Synth is a fully synthetic, high-performance, readily biodegradable, non-toxic hydraulic fluid made from saturated esters. It is combined with high-grade additives, is zinc-free and is environmentally friendly. It leaves no sheen when spilled on water, but appears as visible light foam for easy cleanup. It can resist oxidation at high temperatures and pressures, and prevents gumming and deposits in a hydraulic system. Panolin does not affect conventional sealing materials and provides high corrosion resistance and wear-protection.
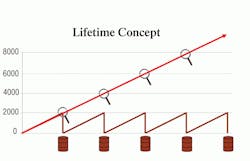
Perhaps the most impressive aspect of Panolin HLP Synth is its lifetime-fill capability. Due to its base composition and saturated nature, Panolin does not break down over time. Thus, this fluid is able to last within a hydraulic system for extremely long periods of time without any oil change requirement, decreasing downtime and maintenance costs. Test documentation shows that some users have been using Panolin in their machines for more than 130,000 hr (that’s operating 24 hr/day per day for nearly 15 years) without changing the fluid, as demonstrated in Figure 3.
Panolin has been used globally for decades in applications ranging from railroads to injection molding machines, and cranes to offshore platforms. Its use is growing in the U.S. as lower maintenance costs and environmental regulations become increasingly important.
Not all fluids are created equal, and it is important to understand the differences when selecting your biofluid.
Contact the author at (713) 680-1951 or email [email protected].
Sources:
1Sauer Danfoss. "Hydraulic Fluids & Lubricants: Technical Information." Sauer-Danfoss.com. Sept. 2010. Web. 26 Sept. 2011.
2 Ruch, Martin. "Panolin Atlantis - Panolin HLP Synth." Message to the author. 24 June 2011. E-mail.
3 Bosch Rexroth Group. "Environmentally Acceptable Hydraulic Fluids." BoschRexroth.com. Web. 26 Sept. 2011.